What are different types of Magnetic materials?
Faraday has divided materials into three classes based on their magnetic behavior. These are –
- Diamagnetic Materials.
- Paramagnetic Materials.
- Ferromagnetic Materials.
Diamagnetic materials
The materials which are weakly magnetized in a direction opposite to the direction of the applied magnetic field are known as diamagnetic materials.
Examples –
Gold, silver, copper, zinc, lead, bismuth, mercury, diamond, marble, glass, silicon, quartz, water, alcohol, air, helium, argon, hydrogen, nitrogen, salts like sodium chloride etc. are diamagnetic materials.
Properties of Diamagnetic materials.
- A magnet repels a diamagnetic material. A freely suspended diamagnetic rod, sets itself at right angle to the direction of external magnetic field.
- Magnetic field lines do not prefer to pass through diamagnetic substances.
- Magnetizing a diamagnetic material is very difficult. They magnetize in a direction opposite to the direction of applied magnetizing field.
- These materials require very strong magnetic fields to show magnetic properties.
- Magnetic behavior of diamagnetic materials does not depend upon change in temperature.
Electron Theory of Diamagnetism
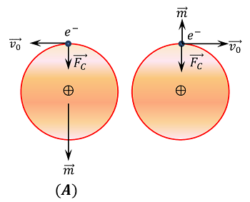
Consider about figure ( A ). In diamagnetic materials, electrons exist in pairs each having orbital motion velocity ( v_0 ) in opposite directions. The magnetic moments of both the electrons are equal and opposite in direction. Hence in the absence of external magnetic field the net magnetic moment of each atom is zero.
Now, let an uniform magnetic field ( B ) is applied perpendicular to the plane of the rotation of electrons i.e. going into the plane of paper.
Due to magnetizing field, an additional magnetic force [ F_m = e ( v \times B ) = e v B ] acts on each electron. For electron, which is revolving in anticlockwise direction, this force is opposite to the centripetal force ( F_c ) . Thus, velocity of electron decreases to ( v_1 ) . For electron, which is revolving in clockwise direction, this force is towards the centripetal force ( F_c ) . Thus, velocity of electron increases to ( v_2 )
Thus a difference in magnetic moments arises in the paired electrons.
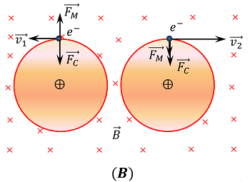
This difference in magnetic moments of paired electrons produces a net magnetic moment. This is called induced magnetic moment. It is perpendicular to the plane of the rotation of electrons i.e. coming out of the plane of paper.
Thus we see that, induced magnetic moment is in a direction opposite to the direction of the applied magnetic field. This is the reason that a diamagnetic substance gets weakly magnetized.
The direction of magnetization is in a direction opposite to the direction of the applied magnetic field.
TO BE NOTED –
- Almost all materials exhibit diamagnetism.
- Susceptibility of diamagnetic substance is directly proportional to the atomic number.
- Susceptibility of diamagnetic substance is independent of the temperature.
Paramagnetic materials
The materials which are weakly magnetized in the direction of applied magnetic field are known as paramagnetic materials. These materials are weakly attracted by a magnet.
Examples –
Aluminium, chromium, manganese, platinum. calcium, antimony, lithium, magnesium, tungsten, copper chloride, salt solutions of iron and nickel, liquid oxygen etc. are paramagnetic materials.
Properties of Paramagnetic materials
- Force of attraction between a magnet and a paramagnetic material is very weak.
- A freely suspended paramagnetic rod slowly sets itself along the direction of external magnetic field.
- Some of the magnetic field lines prefer to pass through paramagnetic substance.
- It is very difficult to magnetize a paramagnetic material.
- Paramagnetic materials require very strong magnetic fields to show magnetic properties.
- Paramagnetic substance get magnetize in the direction of applied magnetizing field.
- Magnetic behavior of paramagnetic materials decreases with the rise in temperature.
Electron Theory of Para-magnetism
Electrons of atoms of paramagnetic substance have orbital motion and spin motion around their axes. The vector sum of these two motions decide the net magnetic dipole moment. Thus atoms of paramagnetic materials have permanent magnetic dipole moment. These are called atomic dipole.
Consider about figure ( A ). The atomic dipoles are oriented in random fashion cancel the magnetic moments of each other. Hence in the absence of external magnetizing field, the net magnetism of material is zero.
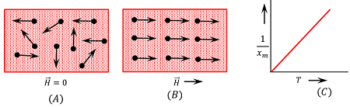
Now, consider that a magnetizing field is applied to a sample of paramagnetic material as shown in figure ( B ). Due to effect of magnetizing field, atomic dipoles try to align themselves in the direction of the applied field. At ordinary temperature, the atomic dipoles fail to attain significant alignment and thus the material gets weakly magnetized. Alignment of atomic dipoles is further disturbed with the rise in temperature of material.
Thus, paramagnetic material loses their magnetism with rise in temperature i.e. their magnetic susceptibility decreases with increase in temperature.
Variation of \left ( \frac {1}{x_m} \right ) with absolute temperature ( T ) for a paramagnetic substance is shown in figure ( C ).
Ferromagnetic materials
The materials which are strongly magnetized in the direction of the applied magnetizing field are known as Ferromagnetic materials.
Examples –
Iron, steel, nickel, cobalt and alloys like AlNiCo (Aluminium + nickel + cobalt) are materials.
Properties of Ferromagnetic materials.
- Ferromagnetic substances can be easily magnetized.
- A freely suspended ferromagnetic rod quickly sets itself along the direction of external magnetic field.
- Magnetic field lines prefer to pass through ferromagnetic substances.
- Ferromagnetic effect is noticed even in the presence of weak magnetic field.
- With the rise in temperature, it becomes comparatively less easier to magnetize the ferromagnetic substance.
Electron Theory of Ferromagnetism
A domain is a small region formed by a large number of atoms of a ferromagnetic substance. Atoms of ferromagnetic substance behave as small magnetic dipoles. Electron of one atom interacts with electron of the nearby atom by the way called exchange interaction or coupling.
In a domain, coupling effect arranges the atoms in a particular direction. Each domain thus behaves as a tiny magnet having some magnetic dipole moment.
When there is no external magnetic field, the magnetic moment of domains are oriented in different directions such that net magnetic moment of the material is zero as shown in figure ( A ).
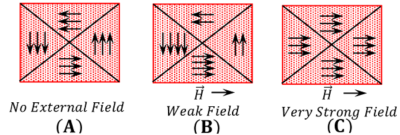
When a magnetizing field is applied, the magnetic moments of domains align in the direction of the applied magnetic field.
The magnetic moments of domains arrange in the following manner –
( 1 ) Arrangement of boundaries – In the presence of weak magnetizing field, the boundaries of domain having magnetic dipoles in the direction of field are increased and opposite to the direction of field are decreased as shown in figure ( B ).
( 2 ) Swinging effect – In the presence of strong magnetizing field, the domains start swinging around such that they all set in the direction of applied field. Ultimately all the domains behave as merged into one another as shown in figure ( C ). As a result, the material strongly magnetized in the direction of the applied magnetic field.
If temperature is increased, the arrangement of domains again disturbed and material begins to lose magnetism. Thus, at a particular temperature ( i.e. Curie ‘s point ) ferromagnetic substances start behaving as a paramagnetic substances. For example at about 1000 \ K , iron behaves as a paramagnetic material.
Types of Ferromagnetic materials.
Ferromagnetic materials are of two types –
- Hard ferromagnetic materials.
- Soft ferromagnetic materials.
| Hard Ferromagnetic materials : | Soft Ferromagnetic materials |
|
|
Example –
| Example –
|
