What is Calorimetry?
Calorimetry is the branch of physics which deals with the measurement of heat addition or rejection from a body or system.
- Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transfer associated with changes of state, phase change or chemical reaction.
- In the laboratory, the process of calorimetry uses a device called a calorimeter.
- A calorimeter is a device used to measure the quantity of heat transferred to or from an object.
Principle of Calorimetry
The principle of calorimetry states the law of conservation of heat energy.
It states that the heat gain by a cold body is equal to the heat loss by the hot body if there is no loss of heat to the surroundings.
Therefore, by the principle of calorimetry –
\text {Heat gain by cold body} = \text {Heat loss by hot body}
Specific Heat
Specific heat of a substance is defined as the amount of heat required to raise the temperature of unit mass of the substance by one degree.
Let, ( \Delta Q ) is the quantity of heat required to raise the temperature of ( m ) mass of a substance through ( \Delta T ) .
Then specific heat will be –
c = \left ( \frac { \Delta Q }{ m \Delta T } \right )
Molar Specific Heat
Molar specific heat of a substance is defined as the amount of heat required to raise the temperature of one mole of the substance through one degree.
Consider about mass ( m ) of a substance of molecular mass ( M ) .
Then, number of moles of the substance will be –
n = \left ( \frac {m}{M} \right )
Let, ( \Delta Q ) is the quantity of heat required to raise the temperature of ( n ) moles of the substance through ( \Delta T ) .
Then by definition, molar specific heat will be –
C = \left ( \frac { \Delta Q }{ n \Delta T } \right )
Or, \quad C = \left [ \frac { \Delta Q }{ ( m / M ) \Delta T } \right ] = M \left [ \frac { \Delta Q }{ m \Delta T } \right ]
= M c
Therefore, \quad \text {Molar Specific Heat} = \text {Molecular mass} \ \times \ \text {Specific heat of body}
Heat Capacity or Thermal Capacity
The heat capacity or thermal capacity of a body is defined as the amount of heat required to raise the temperature of that body through one degree.
Heat capacity or thermal capacity plays an important role in calorimetry process.
- Before going to any experiment for measure of specific heat of a substance, heat capacity of the calorimeter must be known.
- For a calorimeter, it is determined by experimental process by mixing of cold and warm water process.
Let, ( S ) is the heat capacity or thermal capacity of a body whose mass is ( m ) and specific heat is ( c ) for the material of the body.
Then by definitions of specific heat and thermal capacity we find –
S = ( m \times c )
Therefore, \quad \text {Heat Capacity} = \text {Mass} \ \times \ \text {Specific heat of body}
CGS unit of heat capacity is Calorie per degree Celsius and SI unit is Joule per degree Kelvin.
Water Equivalent
The water equivalent of a body is defined as that quantity of water whose temperature can be raised by ( 1 \degree C ) by the the same amount of heat as is required by the body to rise its temperature by ( 1 \degree C )
In CGS system, the unit of water equivalent is gram and in SI system, it is kilogram.
Consider about a body of mass ( m ) gram and specific heat ( c ) .
Then heat required to raise temperature of body by ( 1 \degree C ) , will be –
Q = ( m \times c ) calorie.
Or, \quad m = \left ( \frac {Q}{c} \right )
We know that for water, specific heat is ( 1 \ \text {calorie} \ \text {gram}^{-1} \ \degree C^{-1} ) .
Therefore, mass of water whose temperature can be raised through ( 1 \degree C ) by heat quantity of ( Q ) calorie will be –
m = \left ( \frac { Q }{ 1 } \right ) = Q gram of water.
Therefore, water equivalent of the body will be expressed as –
w = Q \ \text {gram}
Or, \quad w = mc \ \text {gram}
Hence, \quad \text {Water equivalent of body in gram} = \text {Mass of body in gram} \ \times \ \text {Specific heat of body material}
Change of State
A matter can exist in three different states. These are solid, liquid and gas. One state of a substance can be changed to other state by heating or cooling.
The transition of a substance from one state to another state is called change of state.
Melting Point
The fixed temperature at which a solid substance melted to transform into liquid state is known as melting point.
Melting point temperature of a substance has following characteristics –
- Melting point is the temperature at which solid and liquid states of a substance coexist in thermal equilibrium.
- It is the fixed temperature which remains constant till whole solid substance melts into liquid.
- At this temperature, the quantity of heat added to the system is used as latent heat of fusion.
- Melting point increases with increase in pressure for those substances which expand on melting e.g. paraffin wax, phosphorus, sulfur etc.
- Melting point decreases with increase in pressure for those substances which contract on melting e.g. ice, cast iron, bismuth etc.
Boiling Point
The fixed temperature at which a liquid substance boils to transform into vapor or gas state is known as boiling point.
Boiling point temperature of a substance has following characteristics –
- Boiling point is the temperature at which liquid and vapor states of a substance coexist at thermal equilibrium.
- It is the fixed temperature which remains constant till whole liquid substance vaporizes to vapor.
- At this temperature, the quantity of heat added to the system used as latent heat of vaporization.
- Boiling point of liquid substances increases with increase in pressure.
Regelation
When pressure increases, melting point of ice decreases and ice get melts. When pressure is released melting point normalize to initial value and ice again get freezes.
The phenomenon of ice melting upon increase in pressure and freezing again upon relieving of pressure is called Regelation.
- The effect of pressure on freezing point of ice is the cause of regelation.
- Volume of ice decreases when it melts. Hence, its melting point decreases with increases in pressure.
When two blocks of ice are pressed against one another for few seconds and then released, the two pieces found joined together. As the pressure on ice is increases, the melting point of ice decreases at contact surface of ice blocks and ice get melts. When pressure is released the water so formed at the contact surface get immediately freezes again and thus two blocks unites as a single piece.
Application of Regelation
- When the wheels of a cart pass over snow the ice melts due to increase in pressure by the wheels. When pressure is released, water so formed refreezes on the wheels. So wheels are covered with ice.
- Skating is possible due to the formation of water layer below the skates. Water is formed due to the increase of pressure which acts as lubricant.
- The ice of a glacier pressed against the sides of its valley melts and in this way adopts itself to the shape of the valley.
Latent Heat
Latent heat of a substance is that quantity of heat in the phase change process which is not sensed by a thermometer.
Consider that a lump of ice at temperature ( - 5 \degree \ C ) is being heated at normal temperature and pressure. The container is fitted with a thermometer.
- On addition of heat, first the temperature of ice will rise from ( - 5 \degree \ C \ \text {to} \ \text 0 \degree \ C ) . Quantity of heat added at this stage is sensed by the thermometer showing increase in reading. This quantity of heat added to the ice is called sensible heat.
- On further heating, the ice starts melting at fixed temperature of ( 0 \degree \ C ) . This temperature remain fixed till the whole ice melts into liquid form. Hence, quantity of heat added at this stage is not sensed by thermometer. It is also called insensible heat or Latent heat.
Types of Latent Heat
Based upon the type of phase changes, latent heat is of four types –
- Latent heat of fusion or melting ( Phase change from solid to liquid state. )
- Latent heat of vaporization ( Phase change from liquid to vapor or gaseous state. )
- Latent heat of condensation ( Phase change from gas or vapor to liquid state. )
- Latent heat of solidification or freezing ( Phase change from liquid to solid state. )
Now, consider about the heating of ( 1 kg ) of ice at ( - 20 \degree C ) . Temperature verses heat diagram for heating effect is shown in the diagram which is self explanatory.
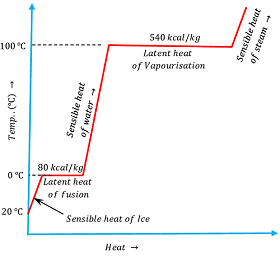
The amount of heat required to change the state of unit mass of a solid substance into liquid state at constant temperature and pressure is called the latent heat fusion of that substance.
In similar manner, the amount of heat required to change the state of unit mass of a liquid substance into gaseous state at constant temperature and pressure is called the latent heat vaporization of that substance.
Consider about ( m ) mass of a solid substance which undergoes a change of state from solid to liquid.
Then, the amount of heat required for this state change will be –
Q = m L
Here, ( L ) is the latent heat of fusion.
Its CGS unit is calorie per gram and SI unit is Joule per kg.
Latent heat of fusion of water at normal pressure is ( 80 ) kilocalorie per kilogram and latent heat of vaporization is ( 540 ) kilocalorie per kilogram.
See numerical problems based on this article.
